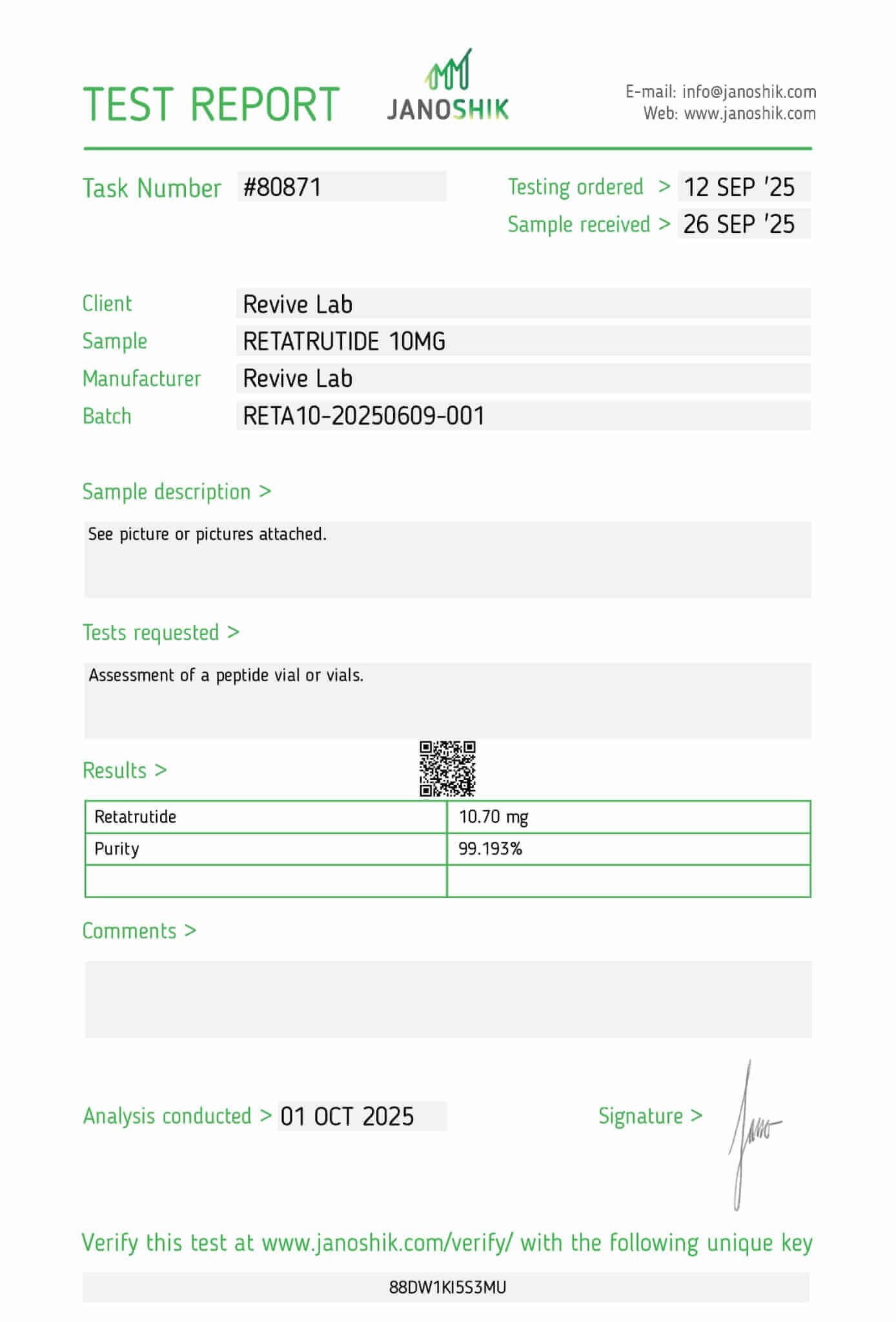
Retatrutide
Retatrutide – Triple Agonist Metabolic Research Peptide
Retatrutide is an experimental triple-agonist peptide targeting the GLP-1, GIP, and glucagon receptors, making it one of the most advanced metabolic research compounds currently under investigation. Early preclinical and clinical data suggest potent effects on energy balance, adipose metabolism, glucose regulation, and body-weight modulation via coordinated incretin-pathway activation. Researchers are exploring its ability to influence appetite signalling, thermogenic pathways, lipid oxidation, and glucose homeostasis with significantly enhanced activity compared to single- or dual-agonist analogs.
$99.99
In stock
Disclaimer: This compound is not intended for human or veterinary use. Retatrutide is sold strictly for laboratory research purposes only. Any mention of effects is provided for educational information and relates solely to preclinical or experimental studies and does not imply efficacy in humans.
Fat Loss & Body Composition
- Promotes unprecedented fat mass reduction via triple receptor activation (GLP-1, GIP, glucagon).
- Induces average weight loss of up to 24.2% in clinical trials—among the highest seen for any injectable peptide.
- Reduces visceral and subcutaneous fat, including in high-risk abdominal and hepatic regions.
- Preserves lean body mass during rapid fat loss, supporting healthy body composition.
- Decreases waist circumference by up to ~19.6 cm, enhancing metabolic outcomes and aesthetic fat reduction.
Appetite Regulation & Caloric Intake
- Strongly suppresses appetite through central GLP-1 and GIP receptor activation.
- Enhances satiety signals post-meal and reduces hedonic food cravings.
- Slows gastric emptying, prolonging fullness and reducing frequency and volume of meals.
- Glucagon receptor agonism adds an independent appetite-suppressing effect, creating a multi-layered reduction in calorie intake.
Glucose Regulation & Insulin Sensitivity
- Improves fasting glucose, HbA1c, and postprandial insulin responses in humans.
- Enhances insulin sensitivity by lowering fat mass and reducing HOMA-IR (insulin resistance index).
- Stimulates glucose-dependent insulin secretion via GLP-1 and GIP pathways.
- Facilitates remission of prediabetes in over 70% of obese trial participants after 48 weeks.
- Reduces fasting insulin and C-peptide by ~50%, indicating improved beta-cell efficiency.
Liver Function & NAFLD/NASH Research
- Reduces liver fat content by up to 80% in metabolic-associated fatty liver disease (MASLD) studies.
- Normalizes liver fat (<5% by MRI-PDFF) in over 85% of participants on high-dose therapy.
- Improves liver enzyme profiles (ALT, AST) and lowers biomarkers of hepatocellular injury and fibrosis (e.g. K-18, Pro-C3).
- Supports liver lipid metabolism through glucagon-driven fatty acid oxidation.
Cardiovascular & Vascular Health
- Significantly lowers systolic and diastolic blood pressure via weight loss and metabolic normalization.
- Improves lipid profiles by reducing LDL cholesterol, VLDL, and triglycerides.
- Modulates adipokines: decreases leptin and increases adiponectin, both of which influence vascular tone and inflammation.
- Supports improved endothelial function and vascular remodeling through metabolic regulation.
Energy Expenditure & Metabolic Rate
- Activates glucagon receptors to increase thermogenesis and basal energy expenditure.
- Counters adaptive metabolic slowdown often seen with weight loss (e.g. reduced resting metabolic rate).
- Increases fat oxidation as measured by lower respiratory quotient (RQ) in preclinical studies.
- Promotes a sustained negative energy balance via dual mechanisms: reduced intake and increased calorie burn.
Inflammation & Adipose Tissue Remodeling
- Reduces chronic, low-grade inflammation associated with obesity, insulin resistance, and fatty liver.
- Improves immune-metabolic cross talk by decreasing pro-inflammatory cytokines (IL-6, TNF-α).
- Remodels dysfunctional adipose tissue to a more insulin-sensitive, anti-inflammatory state.
- Enhances adiponectin secretion, a protective adipokine with anti-inflammatory and insulin-sensitizing effects.
Muscle Preservation & Functional Strength
- Maintains lean muscle mass during extreme fat loss (>20% total weight reduction).
- Designed with moderate glucagon receptor potency (~30%) to avoid excessive amino acid catabolism.
- Avoids sarcopenic-type wasting seen in older or extreme calorie-restricted models.
- Ensures a favorable fat-to-lean mass loss ratio (~75:25) in line with clinically safe standards.
Hormonal Axis & Metabolic Peptide Crosstalk
- Engages the GLP-1, GIP, and glucagon receptor triad to maximize synergy in metabolic regulation.
- Mimics endogenous postprandial incretin activity to support glucose handling, satiety, and energy partitioning.
- Provides insight into future multi-agonist research for treating obesity, diabetes, fatty liver, and cardiovascular syndromes.
- Represents a next-generation incretin-based therapeutic model for holistic metabolic improvement.
To maximize the utility of Retatrutide in experimental models, researchers often combine it with synergistic compounds that enhance energy expenditure, protect muscle mass, or amplify metabolic outcomes. These combinations are used in models of obesity, metabolic syndrome, NAFLD/NASH, and endocrine disorders. Below is a summary of notable Retatrutide synergies validated in preclinical and clinical research:
Retatrutide Synergistic Compounds
| Compound | Mechanism of Synergy | Relevant Research / Notes |
|---|---|---|
| AOD-9604 | GH-fragment peptide promoting lipolysis and fatty-acid oxidation without increasing IGF-1. | Complements Retatrutide’s glucagon-driven thermogenic effects; enhances adipose mobilization in metabolic studies. |
| 5-Amino-1MQ | NNMT inhibitor that elevates NAD⁺ and activates SIRT1; boosts metabolic rate and insulin sensitivity. | Works additively with Retatrutide’s incretin signaling to enhance energy expenditure and glucose control. |
| MOTS-c | Mitochondrial peptide activating AMPK and improving glucose uptake in skeletal muscle. | Synergizes with Retatrutide’s GLP-1/GIP effects on insulin sensitivity and mitochondrial biogenesis. |
| CJC-1295 (No DAC) | GHRH analog that increases GH and IGF-1 release; supports lean-mass preservation during caloric restriction. | Balances Retatrutide’s fat-loss effects by sustaining muscle protein synthesis in metabolic models. |
| Ipamorelin | Selective GH secretagogue enhancing pulsatile GH secretion and metabolic rate. | Combined with Retatrutide to optimize body-composition studies (fat loss + muscle retention). |
| BPC-157 | Regenerative peptide that improves angiogenesis and endothelial repair; supports gut and hepatic function. | Protects gastrointestinal and hepatic tissue in models with incretin or glucagon analog administration. |
| TB-500 (Thymosin Beta-4) | Enhances cellular migration and vascular recovery; reduces oxidative tissue damage. | Synergizes with Retatrutide to maintain tissue integrity during metabolic stress or weight-reduction protocols. |
| GHK-Cu | Copper peptide that activates antioxidant genes and improves collagen matrix turnover. | Supports dermal and connective-tissue repair in metabolic or aging-related studies with Retatrutide. |
| Glutathione (GSH) | Core antioxidant peptide maintaining NAD⁺/NADH balance and mitochondrial redox state. | Used with Retatrutide in oxidative-stress and hepatic-function models to enhance detoxification capacity. |
| Thymosin Alpha-1 | Immune-regulating peptide that reduces inflammatory cytokines and improves insulin sensitivity. | Complements Retatrutide’s anti-inflammatory and metabolic effects for systemic balance. |
Potential Research Use Cases for Retatrutide Combinations
- Metabolic & Obesity Models:
Retatrutide + AOD-9604 + 5-Amino-1MQ + MOTS-c
→ Synergistic fat oxidation, NAD⁺ preservation, and improved insulin signaling.
- Muscle Preservation & Body-Composition Studies:
Retatrutide + CJC-1295 (No DAC) + Ipamorelin
→ Enhances GH-IGF-1–driven lean-mass retention during adipose reduction research. - Tissue & Vascular Recovery:
Retatrutide + BPC-157 + TB-500
→ Supports angiogenesis, hepatic repair, and endothelial resilience during metabolic stress. - Tissue Remodeling During Fat Reduction:
Retatrutide + GHK-Cu + BPC-157
→ Promotes tissue repair, skin resilience, and angiogenesis to offset rapid fat mass depletion effects. - Antioxidant & Cellular Protection:
Retatrutide + Glutathione + GHK-Cu
→ Reinforces mitochondrial redox balance and collagen stability in aging-related metabolic models. - Immune & Inflammatory Balance:
Retatrutide + Thymosin Alpha-1 + MOTS-c
→ Promotes immune-metabolic harmony and reduces cytokine stress in metabolic disease research.
Retatrutide (LY3437943) is a triple hormone receptor agonist that targets GLP-1, GIP, and glucagon receptors. This single-chain peptide is engineered for once-weekly administration and is being investigated for potent anti-obesity and anti-diabetic effects. By activating three complementary metabolic pathways, retatrutide has produced large, durable weight-loss and glycemic benefits in research settings, while showing a safety/tolerability profile similar to other incretin-based therapies (primarily mild-to-moderate gastrointestinal effects) (Ref. 7, 8).
Fat Loss and Body Composition Improvements
Dramatic Weight Reduction: Across Phase 2 obesity research, retatrutide produced some of the largest mean weight reductions reported for an investigational anti-obesity agent. In a 48-week trial in adults with obesity, higher doses (8–12 mg) yielded ~22–24% mean weight loss versus ~2% with placebo; >80% of participants achieved ≥15% weight loss, and ~26% on 12 mg achieved ≥30%—a magnitude typically associated with bariatric-level outcomes (Ref. 1).
Reduced Adiposity and Waist Size: The same program demonstrated substantial losses in total fat mass and waist circumference (up to −19.6 cm at 48 weeks), with imaging corroborating reductions in both visceral and subcutaneous abdominal fat depots—key drivers of cardiometabolic risk (Ref. 1, 6).
Improved Body Composition (Fat vs. Lean): Body-composition analyses indicate weight loss with retatrutide is predominantly from fat stores with relative preservation of lean mass. In a DXA substudy in type 2 diabetes, retatrutide significantly reduced total fat mass versus placebo and dulaglutide while the proportion of lean-mass change relative to total weight loss was similar to other modern incretin-based therapies—mitigating concerns about disproportionate lean-mass loss at higher efficacy levels (Ref. 6).
Appetite and Satiety Mechanisms
Enhanced Satiety Signals: GLP-1 and GIP receptor agonism engages hypothalamic and brainstem circuits that raise satiety and lower appetite. Retatrutide mimics post-prandial incretin physiology to suppress energy intake and improve meal-related glycemic responses in a dose-dependent fashion (Ref. 4).
Slowed Gastric Emptying: GLP-1R activation slows gastric emptying; glucagon-receptor signaling can contribute to post-prandial nutrient handling. The tri-agonist design sustains gastric retention, extending fullness and helping reduce overall caloric intake (Ref. 4).
Dual Appetite Suppression: Beyond incretins, partial glucagon-receptor agonism adds an independent appetite-lowering and energy-expenditure signal. Historically, tri-agonists outperform single/dual agonists in preclinical feeding paradigms and energy-balance models (Ref. 5).
Glycemic Control and Insulin Sensitivity
Lower Blood Glucose and HbA1c: In type 2 diabetes, retatrutide produced large HbA1c reductions (~1.2–1.6% beyond placebo at 12 weeks; ~2.0% by 36 weeks at higher doses) while also reducing fasting plasma glucose—outperforming dulaglutide in the Phase 2 head-to-head setting (Ref. 2).
Remission of Prediabetes: In obesity research, a majority of participants with prediabetes reverted to normoglycemia on retatrutide vs a minority on placebo at 48 weeks—consistent with deep improvements in insulin action and weight-related glucotoxicity (Ref. 1).
Increased Insulin Sensitivity: Across trials and substudies, retatrutide is associated with lower fasting insulin/C-peptide and improved HOMA-IR, indicative of enhanced insulin responsiveness and reduced β-cell demand (Ref. 3).
Cardiometabolic and Liver Effects
Blood Pressure Reduction: Systolic/diastolic blood pressure fell on retatrutide, plausibly via weight loss, favorable neurohormonal shifts, and improved endothelial function; these effects paralleled or exceeded those seen with active GLP-1 comparators in the T2D cohort (Ref. 2)
Improved Lipid Profile: Retatrutide lowered atherogenic lipids—LDL-C, VLDL, and triglycerides (with high-dose arms showing robust TG reductions)—while maintaining HDL-C, reinforcing a broad cardiometabolic benefit beyond glycemia and weight (Ref. 1, 9).
Drastic Reduction in Liver Fat (NAFLD/NASH): In a MASLD Phase 2 substudy, retatrutide reduced liver fat by ~80% at 24 weeks, with ~86% of participants normalizing to <5% hepatic fat. Parallel declines in ALT/AST and fibrosis-linked biomarkers (e.g., K-18, Pro-C3) suggest favorable effects on NASH biology (Ref. 3).
Improved Liver Biomarkers: Biomarkers of hepatocellular injury and fibrosis (ALT, AST, K-18, Pro-C3) were significantly reduced in treated subjects, suggesting amelioration of NASH progression.
Overall Cardiometabolic Health: Multi-endpoint improvements—weight, glycemia, BP, lipids, hepatic fat, and inflammatory adipokines—point to system-wide metabolic resilience. Contemporary meta-analytic and narrative reviews synthesize these benefits and contextualize them within multi-agonist biology (Ref. 9).
Energy Expenditure and Metabolic Rate
Increased Thermogenesis: Tri-agonists enhance energy expenditure and browning/thermogenic programs in preclinical models, exceeding GLP-1 alone or GLP-1/GIP co-agonism. Glucagon-receptor activity is a key driver of this thermogenic edge (Ref. 5).
Mitigation of Adaptive Metabolic Slowdown: Sustained, dose-titrated tri-agonism appears to maintain resting energy expenditure during prolonged weight loss, enabling continued loss across 48 weeks in human studies and steady negative energy balance in animal work (Ref. 1, 4).
Higher Fat Oxidation: Mechanistically, partial glucagon-receptor activation augments lipolysis and fatty-acid oxidation, reflected by lower respiratory quotient and hepatic substrate shifts in preclinical/early translation models (Ref. 4, 5).
Muscle Preservation or Lean Mass Effects
Lean Mass Largely Preserved: While any large weight loss includes some lean-mass reduction, DXA data show retatrutide primarily reduces fat mass with proportional lean-tissue preservation similar to other incretin-based agents—despite larger absolute weight losses (Ref. 6).
Balanced Mechanism Avoids Muscle Wasting: Retatrutide was engineered with lower relative potency at the glucagon receptor than at GLP-1/GIP to capture energy-expenditure and hepatic benefits without excessive amino-acid catabolism—an optimization borne out in preclinical receptor-occupancy and translational studies (Ref. 4).
Safety and Tolerability
Adverse Events: The most frequent AEs are GI-related (nausea, vomiting, diarrhea), generally mild-to-moderate and dose/titration dependent; treatment discontinuation rates were low in Phase 2 and overall safety resembled the incretin class profile (Ref. 7, 8).
Cardiovascular and Renal Outlook: Beyond Phase 2 cardiometabolic signals (BP, lipids), a large event-driven Phase 3 cardiovascular/renal outcomes trial (TRIUMPH-OUTCOMES) is underway to test effects on MACE and kidney endpoints in adults with obesity and ASCVD/CKD (Ref. 10).
| Ref. No. | Study / Source | Focus / Key Findings | Link |
|---|---|---|---|
| 1 | Jastreboff A.M., et al. (2023). Triple-Hormone-Receptor Agonist Retatrutide for Obesity. New England Journal of Medicine. | Phase 2 obesity trial: ~22–24 % mean weight loss at 48 weeks; ≥30 % loss in ~26 % on 12 mg; large waist and fat-mass reductions. | PubMed |
| 2 | Rosenstock J., et al. (2023). Retatrutide for Type 2 Diabetes. The Lancet. | Clinically meaningful HbA1c and weight reductions; ~2.0 % HbA1c drop at 36 weeks; active-comparator outperformance. | PubMed |
| 3 | Sanyal A.J., et al. (2024). Retatrutide for MASLD (Phase 2 Substudy). Nature Medicine. | ~80 % liver-fat reduction at 24 weeks; 86 % achieved < 5 % liver fat; ALT/AST, K-18, Pro-C3 improved. | PubMed |
| 4 | Coskun T., Urva S., Roell W.C., et al. (2022). LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metabolism 34(9): 1234-1247.e9. | Discovery and translational optimization of Retatrutide (LY3437943) with balanced GLP-1/GIP plus partial glucagon agonism; strong preclinical and early clinical efficacy. | PubMed |
| 5 | Finan B., et al. (2014). Monomeric GLP-1/GIP/Glucagon Tri-agonist. Nature Medicine. | Foundational tri-agonist concept: greater fat-loss and thermogenesis vs dual agonists in rodents; mechanistic rationale for glucagon activity. | PubMed |
| 6 | Coskun T., et al. (2025). Body Composition (DXA) Substudy. Lancet Diabetes & Endocrinology. | DXA analysis: total fat-mass ↓ significantly by 36 weeks; lean-mass proportion preserved vs placebo/dulaglutide. | PubMed |
| 7 | Katsi V., et al. (2025). Retatrutide — A Game Changer in Obesity Pharmacotherapy. Review. | Comprehensive review of mechanism, clinical efficacy, and safety/tolerability. | PMC |
| 8 | Eli Lilly and Company (2023). Phase 2 Retatrutide Results Press Release (ADA 2023 / NEJM Publication). | Summarized safety profile: GI AEs dose-related, mostly mild; dose-dependent HR increase; foundation for Phase 3 program. | PressRelease |
| 9 | Goldney J., et al. (2025). Triple Agonism Therapies for Obesity: Focus on Retatrutide. Obesity Reviews. | Summarizes Phase 2/3 data, CV/renal potential, and metabolic biomarker improvements. | PubMed |
| 10 | TRIUMPH-OUTCOMES (NCT06383390). ClinicalTrials.gov. | Ongoing Phase 3 cardiovascular and renal outcomes trial testing MACE and kidney endpoints in adults with obesity + ASCVD/CKD. | ClinicalTrials.gov |
Stack With
Retatrutide + NAD⁺
Retatrutide + NAD⁺ + Glutathione

Retatrutide + NAD⁺ + Glutathione + AOD-9604